Brilliant Info About How To Tell If A Reaction Will Occur

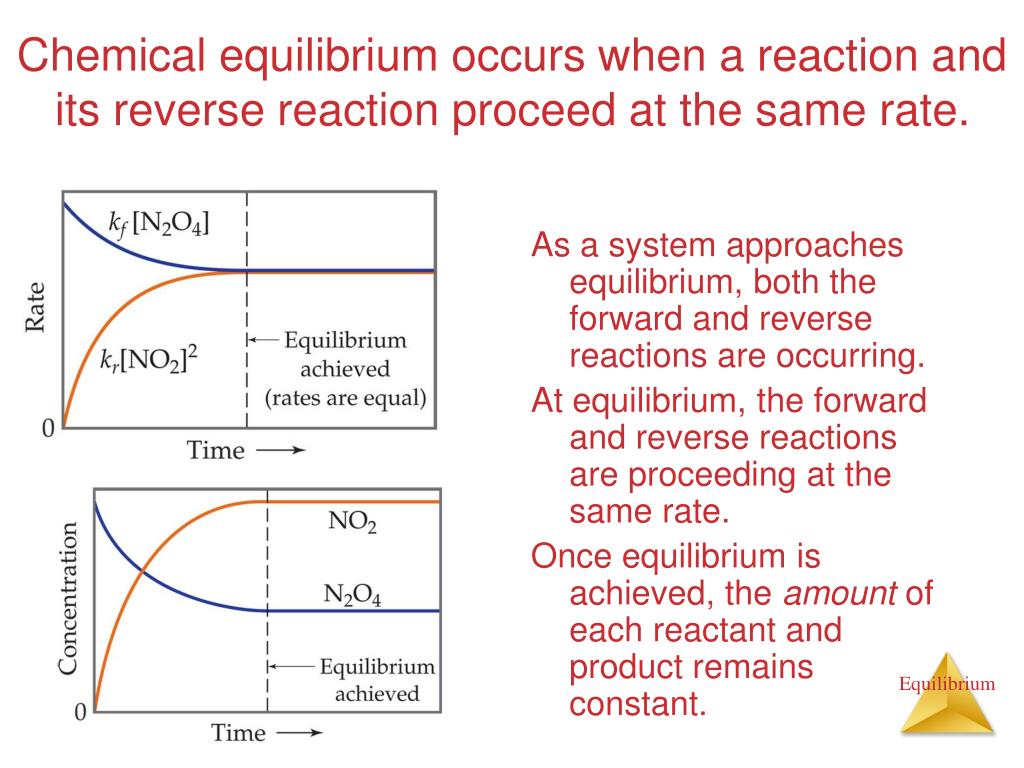
Four methods to determine if reaction is spontaneous or not (∆g, ∆s_universe, e_cell, q vs k) conquer chemistry.
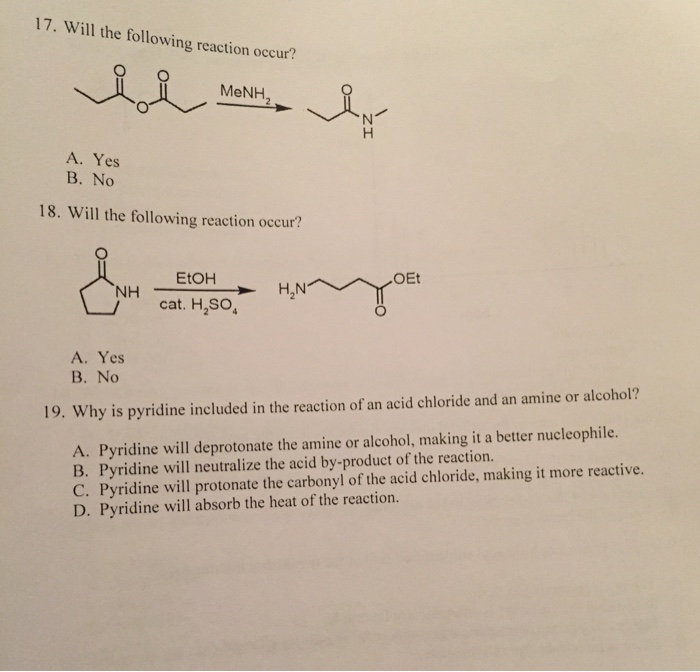
How to tell if a reaction will occur. If q < kₛₚ, the newly mixed. If the reagents in the previous reaction had been $\ce{lialh4}$ followed by $\ce{h3o+}$, then more than one outcome is possible since more than one functional. Recognizing the type of reaction that is occurring is as simple as looking at the given products and reactants in the chemical equation.
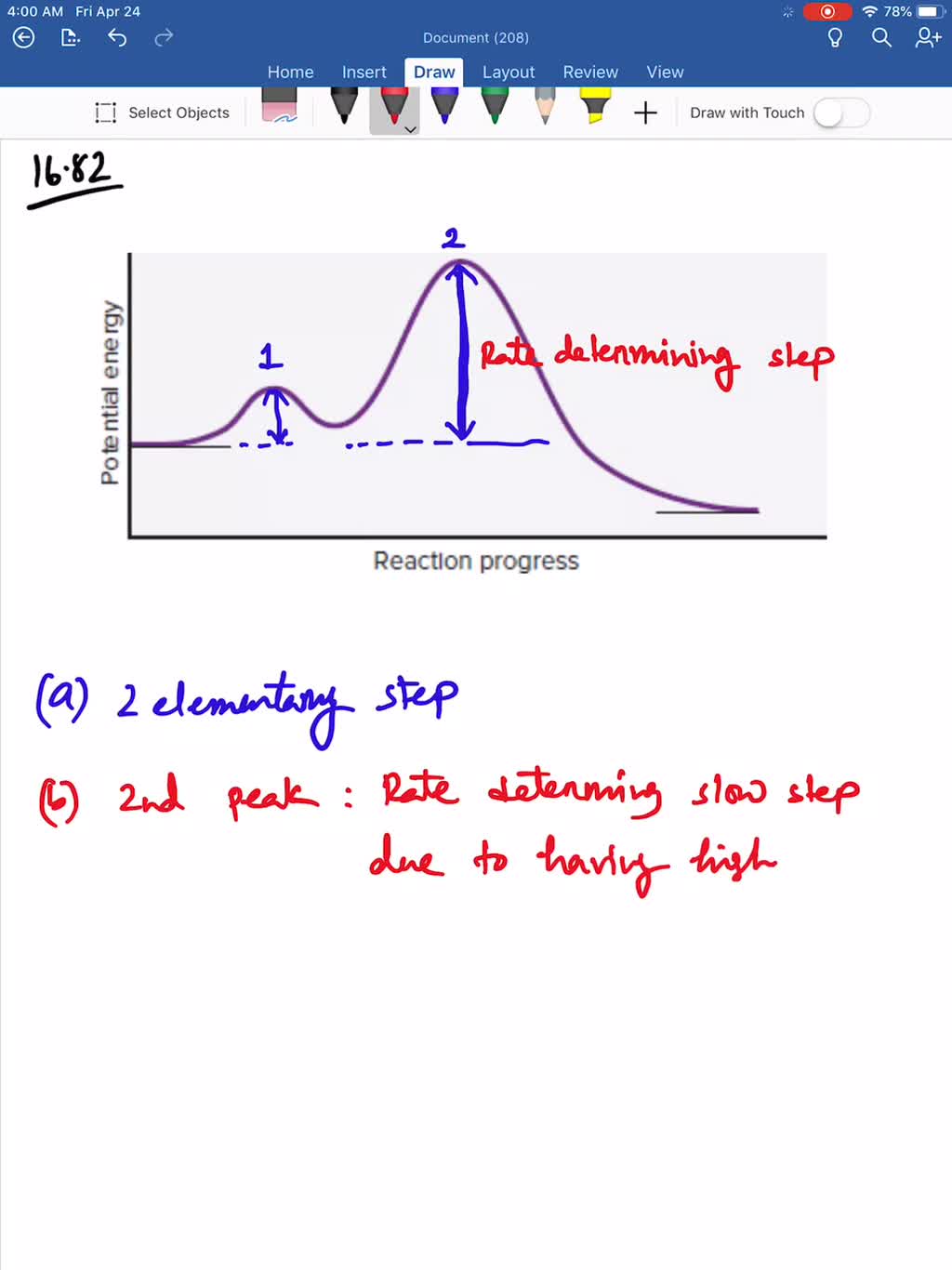
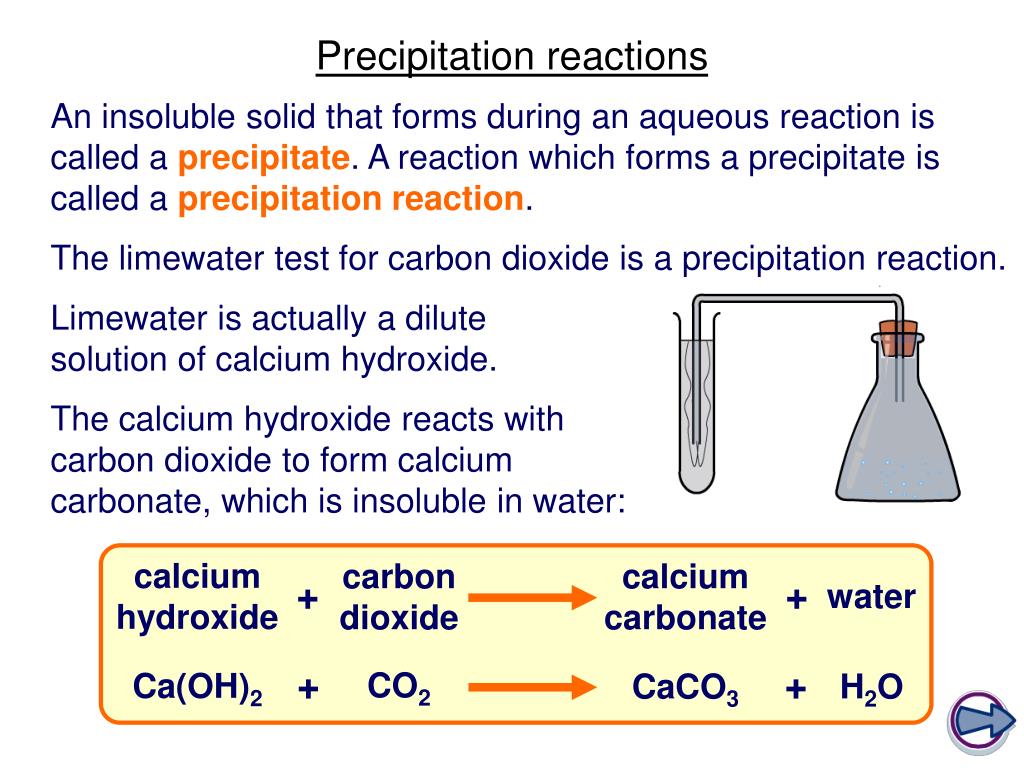
We can use the reaction quotient to predict whether a precipitate will form when two solutions containing dissolved ionic compounds are mixed. A reaction that occurs in two or more elementary steps is. You probably noticed that the products of the above reaction haven't been specified yet.
Click on the nist chemical webbook link under the resources section at the end of this article. Erwin van den burg. A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs.
At&t posted an update on thursday evening, saying that the company does not believe the outage was due to a cyberattack. These reactions are said to be irreversible. Reactions involving neutralization:
This paper attempts to answer the title question in a clear and direct. The reaction would be as follows: For example, solid iron reacting with aqueous sulfuric acid (h 2 so 4).
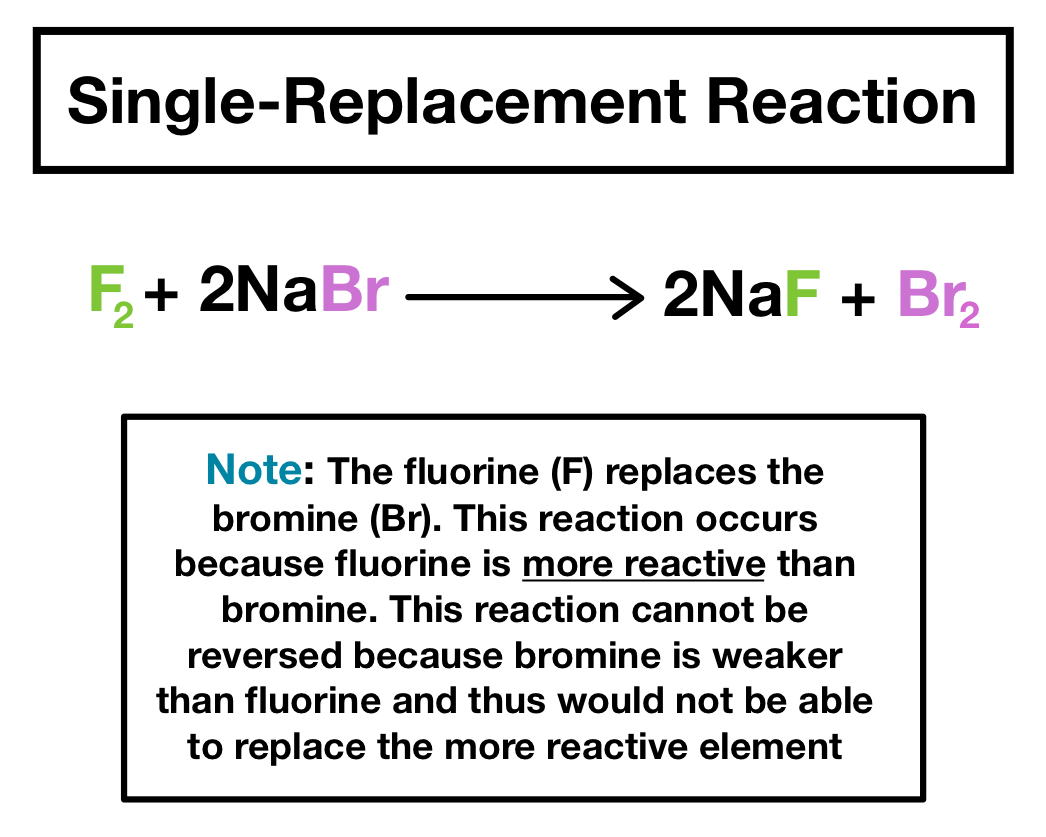
Some chemical reactions simply run in one direction until the reactants are used up. The periodic table or an activity series can help predict whether single. In fact, it is possible the reaction won't happen at all!
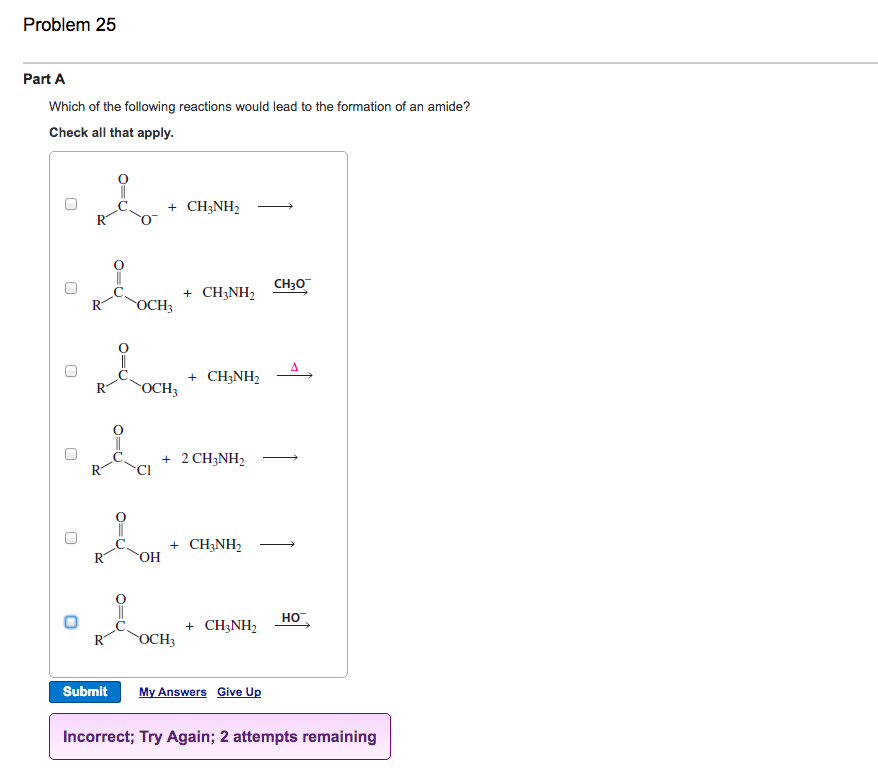
If the reaction does occur, write the products of the reaction and balance the equation. We can also be reasonably confident that no unpleasant side reactions will occur. Use the activity series to predict if the reaction below will occur.
Why should its conjugate acid give off a $\ce{h+}$ to water, if it was a strong. We will figure out in.
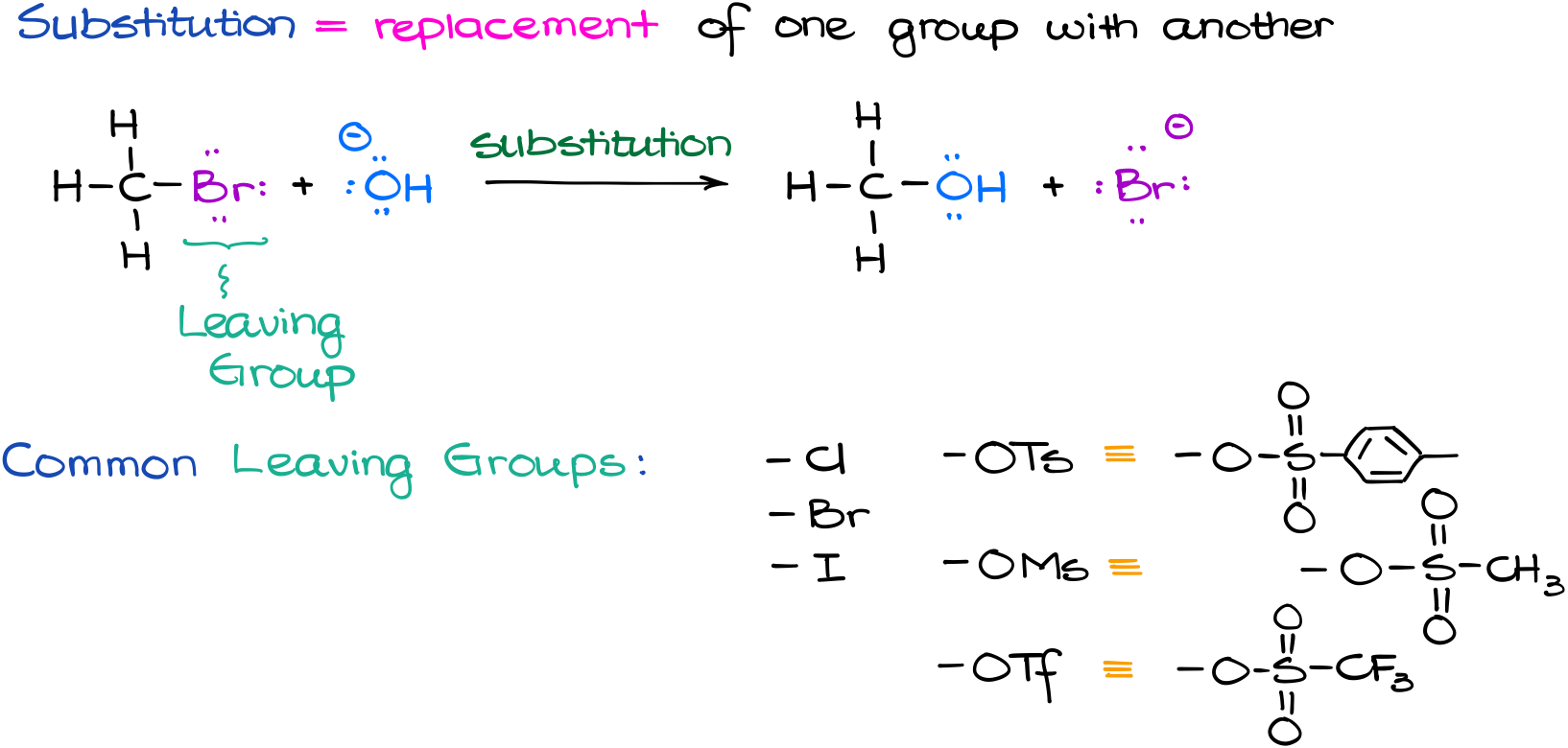
A chemical reaction is the process in which one or more substances are changed into one or more new substances. To determine if a double replacement reaction will occur, you need to look at the products. Why does this reaction occur, because the alkoxide ion is a really strong base right?
If the reaction is predicted to occur, use the same general guidelines that we used above. How can i predict if a reaction will. $\ce{hcl}$ would react with $\ce{(nh4)2co3}$, $\ce{k2so4}$ and $\ce{ba(no3)2}$, as the reactions give out.




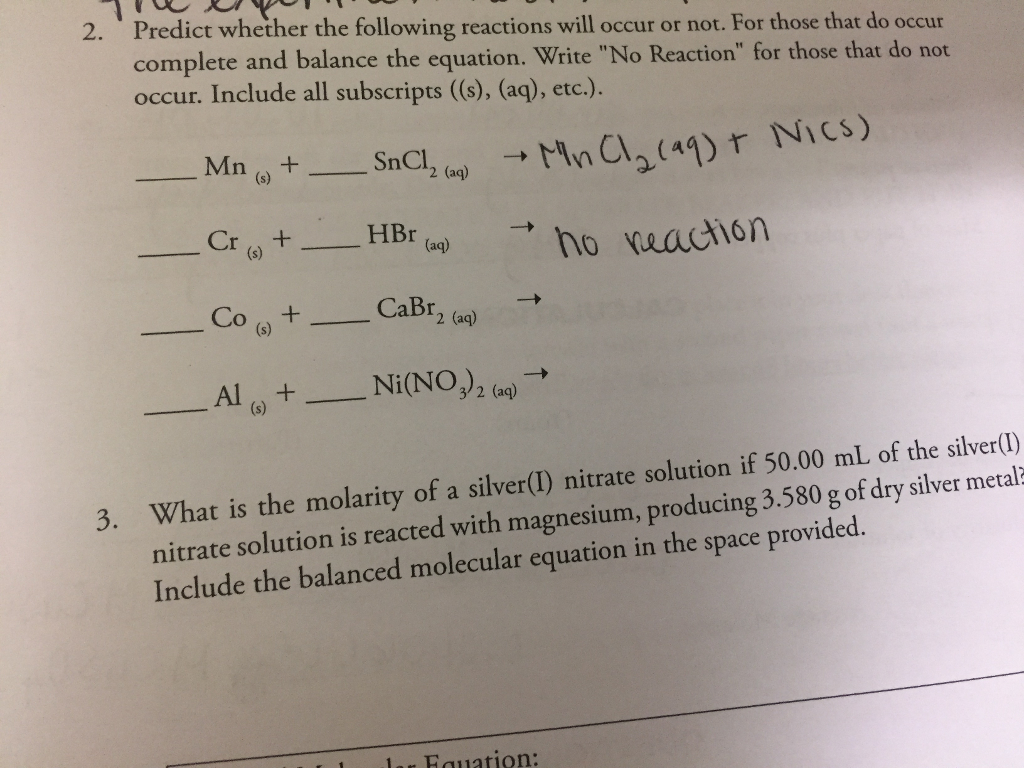

/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)